Abstract
Snails are beautiful creation of nature. They occur in rivers as well
as ponds. But these sources of water are contaminated by
effluents, pollutants, acid rain, particulates, biological wastes etc.
They can change the pH of water. Water is absorber of carbon
dioxide and it converts carbon dioxide into carbonic. Other
above-mentioned wastes also increase the concentration of H+ ions
in water. They produce hostile environment for snails. The outer part of
snails is made of CaCO3. It produces chemical reaction in
acidic medium and corrosion reaction is accelerated thus deterioration
starts on the surface of snails. This medium their survival
becomes miserable. For this work corrosion of snails study in the pH
values of water is 6.5 in H2CO3 environment. The corrosion
rates of snails were calculated by gravimetric methods and potentiostat
technique. Aloe Vera was used for corrosion protection
in acidic medium. The surface adsorption phenomenon was studied by
Lungmuir isotherm. Aloe Vera formed thin surface film on
the interface of snails which adhered with chemical bonding. It
confirmed by activation energy, heat of adsorption, free energy,
enthalpy and entropy. The results of surface coverage area and
inhibitors efficiency were indicated that Aloe Vera developed strong
protective barrier in acidic medium.
Keywords: Corrosion; Snails; Aloe vera; Carbonic acid; Potentiostat; Thin film formation
Introduction
Corrosion occurs in living organisms [1]. The animals’ outer
layer is created by calcium carbonate [2] to corrode in acidic
environment. Corrosive substances interact with living organism
[3] to produce corrosion cell which is exhibited autoredox with
snails [4] and disintegrated their outer layers. It observed that
carbon dioxide [5,6] reacts with water to form carbonic which
produce hostile environment [7] for snails and [8] Ocean water [9]
is major absorber of carbon dioxide to change pH. Carbonic acid
interacts with snails to exhibit chemical thus calcification [10]
starts on their surface. The oxides of Sulphur [11] dissolve in water
to produce sulphrous and sulphuric acid. These acids produce
corroding [12] effect with snails. Oxides of nitrogen [13] absorb
water to form nitrous and nitric acids and they generate corrosive
environment for molluscs [14] Acid rain [15] can change pH of water
and produce acidic medium for snails. Industrial wastes and human
wastes contaminate water sources and alter the pH values of water
in this way it makes water corrosive for snails and molluscs. The
temperature [16] of the earth is increasing due to global warming
thus water sources temperature is also increased and snails [17]
undergo corrosion reaction. Various types of techniques use for
corrosion protection [18] like anodic and cathodic protection,
galvanization and electroplating, dipping [19] anodization, spray,
nanocoating and inhibitors action. Aloe Vera is used for skin
corrosion protection in acidic environment. Snails’ corrosion [20]
can be control by inhibitor action of Aloe Vera in above mentioned
environment. Aloe Vera form a thin barrier on the surface of snails
and it is confirmed by activation energy, heat of adsorption, free
energy, enthalpy and entropy and these thermal parameters results
is noticed that Aloe Vera has good inhibition properties in acidic
medium. It forms complex barrier on the surface of snails.
Experimental
Snails dipped into carbonic acid solution which pH value was
6.2. The corrosion rates of snails were determined by gravimetric
method at mentioned periods 1,2,3,4 and 5 years at 288,298,303,308
and 3130K temperatures without use of Aloe Vera. Aloe Vera was
used as inhibitor in carbonic acid medium and the calculated of
corrosion rate of snails above mentioned years and temperatures at
50, 60, 70, 80 and 90M concentrations. Potentiostat 324 model used
to determine the corrosion potential, corrosion current density at
different temperatures and concentrations. These results were
obtained by application of calomel electrode as auxiliary electrode
and Pt reference electrode. The snail kept between these electrode
and external current passed through without and with inhibitor.
The results were noticed that anodic current decreased and
cathodic current increased by the use of Aloe Vera. The gravimetric
method corrosion rate results were approximated to potentiostat
corrosion obtained results.
Results and Discussion
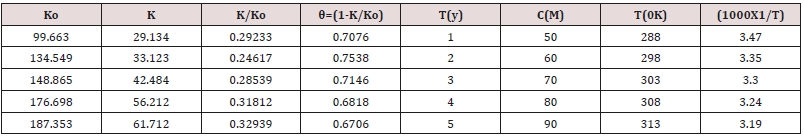
The corrosion rate of snails were determined by without and
with Aloe Vera in mpy (miles per year) at different temperatures,
concentrations and times in years by the use of formula
K=534XΔW/D A t (where ΔW is weight loss in g, A is area in sq
inch, t is immersion time in year). The dipping times were 1,2,3,4
and 5 years and temperatures are 288,298,303,308 and 3130K
without inhibitors corrosion rate of snail is calculated and their
values were recorded in Table 1. The addition of Aloe Vera in
carbonic acid medium and corrosion rate of snail calculated at
288,298,303,308 and 3130K temperatures and 50, 60, 70, 80 and
90M concentrations and its values were mentioned in Table 1. It
observed that without action of inhibitor corrosion rate of snail
increased as duration of times and temperatures were increased
and, but its values were decreased after addition of Aloe Vera
such types of trends noticed in Figure 1 K Vs t, Figure 2 K Vs T and
Figure 3 K Vs C. The surface coverage area and inhibitor efficiency
were calculated by formula θ= (1-K/Ko) and %IE= (1-K/Ko) X100
(where Ko corrosion rate without inhibitor and K corrosion rate
with inhibitor) and their values were given in Table 2. The surface
coverage area and inhibitor efficiency were calculated by formula
θ= (1-K/Ko) and their values were given in Table 2. The results
of Table 2 were shown that surface coverage area and percentage
inhibitors efficiency were enhanced when inhibitors added at
different temperatures and concentrations as per year. Such types
of trends were noticed in Figure 4 θ Vs T and Figure 5 θ Vs C.
Figure 1: K Vs t for snails at different years.
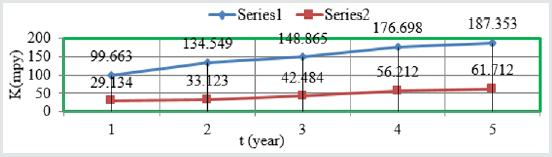
Figure 2: K Vs T for snails at different tempertaures.
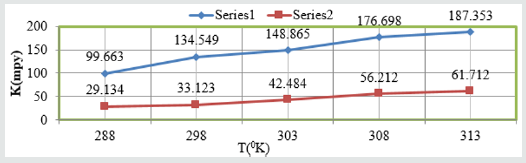
Figure 3: K Vs C for snails at concentations.
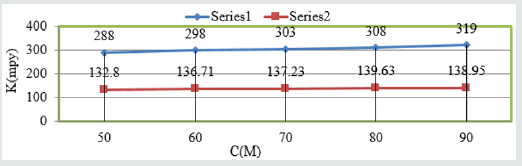
Figure 4: θ Vs T for snails in Aloe Vera.
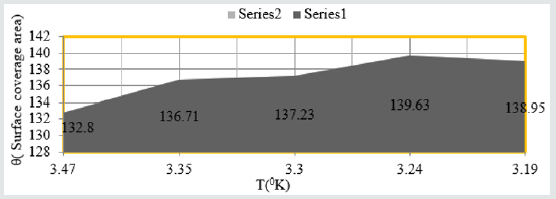
Figure 5: θ Vs C for snails in Aloe Vera.
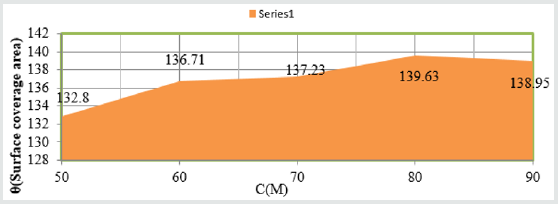
Table 1: Corrosion rate of snail absence and presence of Aloe Vera in H
2CO
3.
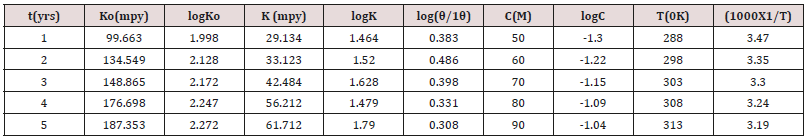
Table 2: Surface coverage area develop by Aloe Vera on the snails in H
2CO
3.
The percentage inhibitors of Aloe Vera at different temperatures
and concentrations as one-year interval were calculated by %IE=
(1-K/Ko) X100 (where Ko corrosion rate without inhibitor and K
corrosion rate with inhibitor) and the values were written in Table
3. The results of Table 3 were depicted that percentage inhibitors
efficiency were increased as temperatures and concentration
were enhanced. Such types of trends also observed in Figure 6
%IE Vs T and Figure 7 %IE Vs C. Surface adsorption phenomenon
was studied by activation energy, heat of adsorption, free energy,
enthalpy and entropy. Activation energy was determined by
formula K=A e-Ea/RT (where K is corrosion rate, Ea is activation
energy and T is absolute temperature without and with action of
Aloe Vera at different temperatures and concentrations and their
values were recorded in Table 4. It observed that activation energy
increased without inhibitors but its values decreased after addition
of inhibitors. These results were shown in Table 4 which indicated
that inhibitors adhered on snails by chemical bonding and their
values were obtained by Figure 8 plotted logK Vs 1/T. Heat of
adsorption values were found to be negative which indicated that
Aloe Vera was shown an exothermic reaction in H2CO3 medium. It adsorbed on the surface of snail by chemical bonding. The values
of heat of adsorption were determined by Langmuir isotherm
log(θ/1-θ) = logA +logC-q/2.303RT and Figure 9 plotted log(θ/1-θ)
Vs1/T and Figure10 plotted against log(θ/1-θ) Vs logC and their
values were recorded in Table 4.
Figure 6: %IE Vs T for snails in Aloe Vera.
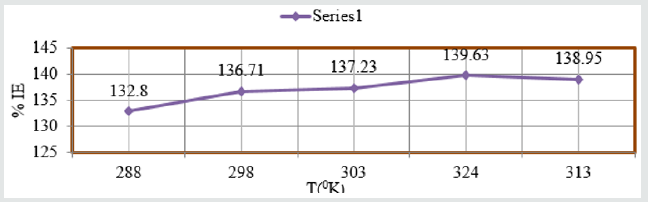
Figure 7: %IE Vs C for snails in Aloe Vera.
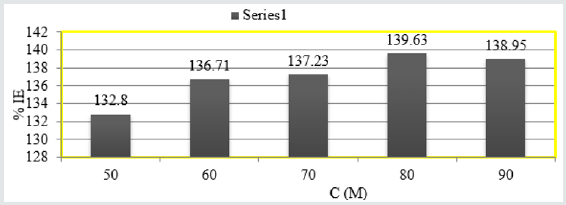
Figure 8: logK Vs 1/T for snails in Aloe Vera.
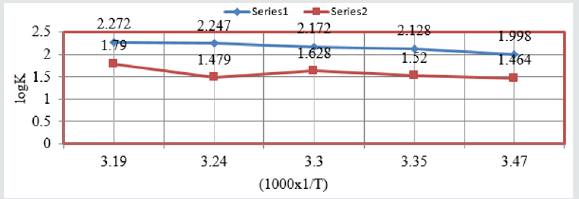
Figure 9: log(θ/1-θ) Vs 1/T for snails in Aloe Vera.
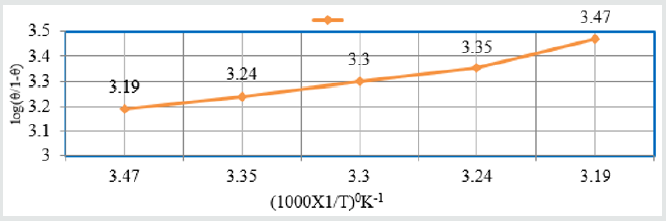
Figure 10: log(θ/1-θ) Vs logC for snails in Aloe Vera.
Table 3: Inhibition efficiency develop by Aloe Vera in H
2CO
3.
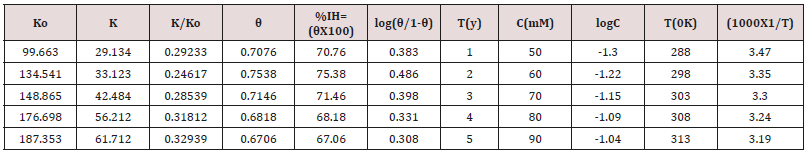
Table 4: Thermal parameters of Aloe Vera with Snails.
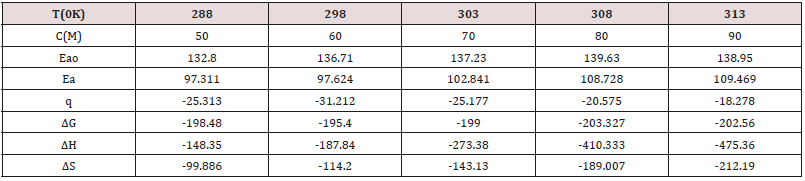
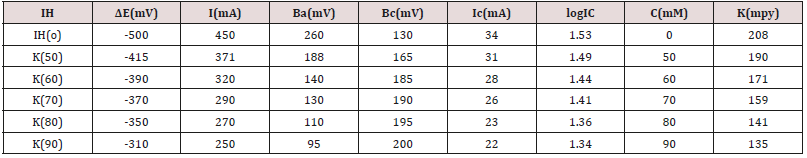
Table 5: Potentiostatic results of snails for Aloe Vera.
Free energy of inhibitor Aloe Vera was calculated by equation
ΔG=2.303 log(33.3K) and their values were given in Table 4. Their
values noticed that inhibitor action a chemical reaction because free
energy values were negative, and their values mentioned in Table
4. Enthalpy of used inhibitors was determined by transition state
equation K=RT/Nh eΔS/R e-ΔH/RT and its values were recorded in
Table 4. These values indicated that inhibitor’s Aloe Vera boned with
snail by chemical bonding. Entropy of Aloe Vera was determined
by equation by ΔG = ΔH – TΔS and their values were mentioned
in Table 4. Their values were shown that deposition of Aloe Vera
on the surface of snail was an exothermic process. It formed
stable barrier on the surface of snail. All five values of thermal
parameters plotted against T in Figure 11 and Figure 12 against C.
The corrosion potential, corrosion current density and corrosion
rate were determined by the equation ΔE/I=1/2.303 βaβc/(βa+βc)
and C R(mpy)=0.1288 Ic (mA/cm2) XE/ρ and values were recorded
in Table 5. It observed that without inhibitor corrosion potential
and corrosion current were decreased but after addition of Aloe Vera corrosion current densities were increased. It also reduced
the corrosion potential and corrosion current. The corrosion
rate calculated by potentiostat technique and their values were
tallied with the corrosion rate determined by gravimetric method.
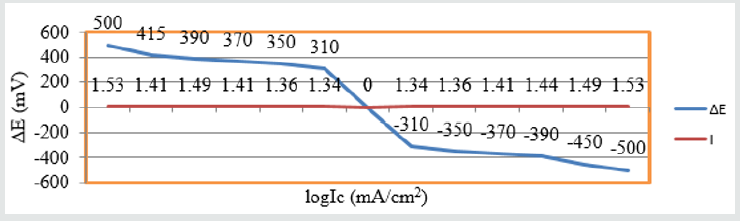
Corrosion potential versus corrosion current density was plotted
in Figure 13. This plot indicated that anodic current reduced as
addition of inhibitor but cathodic current enhanced Table 5.
Figure 11: Themal energies Vs T for Aloe Vera with Snails.
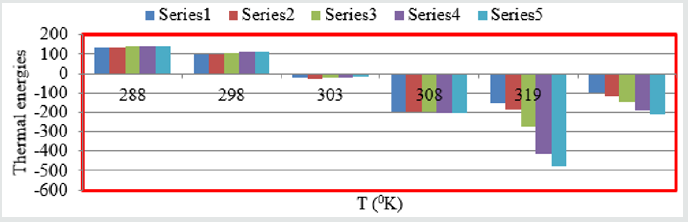
Figure 12: Thermal energies Vs C for Aloe Vera with snails.
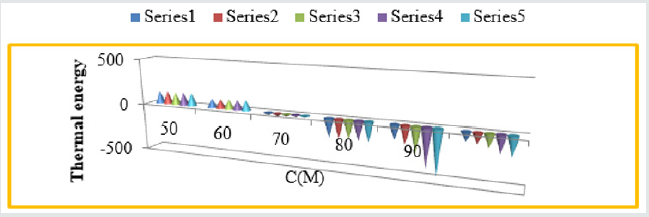
Figure 13: ΔE Vs Ic for snails with Aloe Vera.
Conclusion
Snails’ corrosion occurs due to change the pH of water. Water
pH is altered by contamination effluents, industrial polluters, and
various types of wastes and acids rain. Snails outer layers are
constructed by calcium carbonate. In acidic medium calcification
starts on their surface by chemical process. It produces pitting,
stress and crevice corrosion. For the protection of such types
corrosion Aloe Vera is used as inhibitors. Aloe Vera forms thin
film on the surface of snails. The thin film formation is confirmed
by thermal parameters like activation energy, heat of adsorption,
free energy, enthalpy and entropy. Aloe Vera surface adsorption
phenomenon on snails is also satisfied by Langmuir isotherm.
Aloe Vera is reduced the concentration of H+ ions and enhance
the concentration of oxygen molecules. It is nitrogen containing
rich organic compounds which capture H+ ions and less H2 gas is
released thus corroding effect of snails suppressed.
Acknowledgment
Author is thankful for UGC-New Delhi, India for providing
financial support for this work. I also thank my research team
for their collection of data and graph plotting. I am very grateful
professor G Udhayabhanu IITD and professor Sanjoy Misra
providing laboratory facility.





















No comments:
Post a Comment