Lupine Publishers| Modern Approaches On Material Science
Abstract
Study focuses on the basis of knowledge the mechanism of the process βSn αSn for use it to analysis of important material for science and technology. The possibility of ultra-high purity Sn to analyse by measuring the rate (V) of the allotropic changing (V βSn αSn) is investigated. Metals of such high purity are inaccessible to chemical method, so analyzed by method of a residual resistance at temperature (T) of liquid He, inaccessible to most enterprises. The method gives an estimate of the total content of impurities. For Sn with low T of βSn αSn) due to the simplicity of the measuring purity by the V (βSnαSn) is tempting. In high purity Sn with a low content of impurities, this method seems more accessible and convenient than others and probably possible. This paper proposes the affordable and simple method of analysis, high sensitivity, accuracy and reproducibility of the results. not inferior to the complex method of measuring the residual resistance.
Keywords: Residual Resistance; Phase Transition Rate; Impurities
Introduction
The World made 7 metals, according to the 7 planets. (Navoi). In the table of ranks of the ancient Sn is pair to Jupiter, the largest planet. And now Snwith the honorary № 50 in the center of the Periodic Table of Mendeleev. Sn is the oldest to man known metal. Aristotle knew about the Sn plague, but didn’t know that it was a consequence of the allotropic transformation of Sn white to gray, β®α. The nebulous mysteries of Sn plague infection accumulated interests many centuries tothis phenomenon.A main Interest in βSn®αSn appeared after the evidence [1,2]Goryunova semiconductor nature of αSn with covalent bond by changing the metal bond to covalent, the electronic structure s2 p2βSn to sp3,tetragonal structure with
KN=6 to a cubic structure with KN=4 with bonds to the vertices of tetrahedrons ofαSn.These principlescreating of semiconductor compounds ofneeds properties. To turn into metastable αSn except T below 12.4oC,is a necessary [2] seed withthe parameters of the bond and structures related αSn and its contact with tin.The nearest neighbors of Sn give a compounds InSb and CdTe, There, pairs of atoms give in sum of total electrons the same as 2 atoms of Sn and parameters of structures [1] almost the same of αSn. InSb, CdTe, αSn the better seed of Sn®αSn, but in contrast to metastable αSn powder, InSb, CdTe are strong solid crystals. Theinfection is caused by atomic contact with a seed. Tin always covered by protective film of SnO2which don’t allow contact.If the seed is placed on the surface of Sn, there is Infection!? And from inert substances that had contact previously with the seed although it now removed[3]. Solid crystals recognized the past! Infection at a distance is possible too![4]. It was quite misunderstood: what gives an information from the seed? Necessary presence of the air, atmosphere.There is Ic agent,[5-7] Inthe vacuum, dryvessel, or after treatment of the inert substance with any solvent of water, so there is no infection,Ic is a carrier from the seed. Metastable structure Ic in the size of nanoparticles can growing epitaxially on the related structure, penetrate through the microdefects of the protective SnO2. So, it is clear that infection under water which absorbed the Icnanoparticles is impossible.This opinion turned out to be wrong. With a very small probability for a time more a year under moving water, infection occurs, and this valuable phenomenon gives ways to many practical tasks andunderstanding of life processes[7].The source of infection has been found. and yet another unexpected source of infection was found. This is property for practical aim. Tin remember about stay in the αSn phase. There is a βSn®αSn transition and back αSn®βSn, due to a change of .>/<d by 26.6%.volume effect. At each β®αmovedecreased d and at α®βd increased. So without external tools Sn gives pure powder of any size particles[8,9].
Knowing the Icas seed allows to use for solving a row of other practical problems [5-7]with use of the terrible plague by a simple way [10,11] in forms convenient for creating p/n shifts , simple effective purification of Sn without meltingin solid phase [12].Method of zone melting [13] to purification is determined by the difference in the K, ratio of the solubility of impurities at the phase boundary. At melting metal doesn’t change type of the bond on the border ofsolid/liquid, soK is near to 1, the difference is knowingly less than at of the metal /semiconductorboundary with the great differences in the nature of their chemical bonds, CN (coordination number), structures. The cleaning efficiency at the border metal /semiconductor, K far from1. And so was a reason that zone melting became widely used when there was a need in semiconductors of high purity.A knowledge of the mechanism of the solid-phase process of βSn ®αSn [7] land to opinionof possibility to apply it in the analysis of the height purity of Sn.
Theoretical View on The Possibility of Analyzing by V Βsn→Αsn
Analysis of high-purity materials is labor-intensive and often impossible if the sensitivity of classical methods is insufficient [14]. There is a method for measuring the g4.2К, i.e. the ratio R 300K /R 4.2 K, method of residual resistance, which gives an estimate of the amount of impurities in metals [15] of high purity. The residual resistance of Sn at 4.2 K before the transition to the superconducting state depends on its purity and perfection of structure. The R at T of room is almost constant, and the g4.2К, i.e. the ratio R 300K /R 4.2 K, is residual R characterizes the purity of Sn.The purer the metal and more perfect its structure, the lower the R at 4.2 K and the higher the value g4.2К, which serves as a measure of the total content of impurities in metals. But measuringequipment is difficult, and liquid He is rarely available to the most of organizations. Studies of allotropic transformation of Sn [5-7] showed a connection between the purity by g4.2К, and the rate V of its phase transformation into αSn. But also, it seemed unrealistic to use it for analyses after bright experiments [16] showed the impurities in Sn are accelerating, indifferent and inhibiting. Hence, the analysis of the purity of Sn by V βSn®αSnis impossible at it depends on the ratio of concentrations of dissimilar impurities. But the mechanismof distinguishing the role of impurities is not clear at all. If each atom of the impurity violates the g4.2К, of the metal, which theg4.2Кmethod illustrates by analyzingany other metals, why the impurities of different metals differ in their effect on the V βSn®αSntransition. This became clear when we knew the mechanism of infection with the "tin plague" [4]. In [16] was studied Sn not of high purity, there are no errors in experiments. The chaotic nature of the dependences of V on purity is clearly shown [5,7]atstudying the influence of impurities on V of βSn ®αSn. The fact is that the commonly zone melting is powerless to clean from Sb because it has K=1 in Sn. The solubilities of Sb in solid and molten Sn are the same, And the Sb impurity on both sides of the phase boundary is the same and so can’t to be redistributed, as other impurities with K≠1.And in the ores of Sn impurity in the Sb usually dominates. At zone melting cleaning, the Sb impurityalways prevails over the others. And Inhimself like of all metals is inhibitory too by the same reasons, but it was shown as accelerator [16] because In+Sb gives the best seed InSb. And in the Sn of high purity, the impurity of In, like any impurity, individual. But having the knowledge aboutthe dependence of the βSn ®αSnprocess on many factors, it is necessary to observe the requirements 1-4, understood during the experiments for creating a method for analyzes[17].
Experimental Part
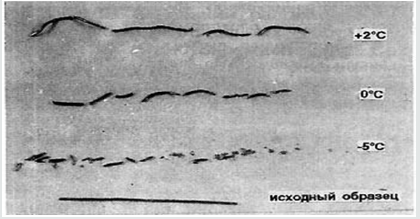
It is possible to create a method for analyzing the purity of V βSn → αSn similar to measurements of residual resistance, suitable for high-purity metals. Previously, it was found [3,5,7] that the dependence of V βSn → αSn on T for any samples has a maximum. This is very easy to understand. At low T with its growth V βSn → αSn grows according to the Arrhenius equation. V cannot grow constantly, because as it approaches the point of the phase transition, it becomes smaller and turns to 0. When infected, Sn crumbles into an arc-shaped powder, making difficult to measure phase shift lengths. Amorphous wires of fast quenching, single crystals of βSn and even annealed wires with slow infection remain almost the original shape but with some bending, and break at V βSn→αSn depending on the T (Figure 1) to parts of different lengths, but almost the same at each T. Accumulation of impurities by the method of residual resistance was recorded in the fracture. It is seen that after the fracture, the sections at each T are close to each other. For analysis, it is necessary that the content of impurities is constant along the length, that is, choose V βSn → αSn for it, V of growth of αSn and V of impurities were now equal, and Sn maintain the solidity too.
Figure 1: Fracture of Sn of different purity with the accumulation of impurities overtaking the phase boundary at its low V. T= +2:0 and -5 ̊С.
Requirements
1) Monoliths are obtained for the growth of αSn [10,11] in the ice shape. The study of a movement of impurities at βSn → αSn allowed us to create a method like of zone cleaning in a solid, but for analysis it is necessary that the content of impurities is constant along the length, that is, choose V βSn→αSn and V of impurities equal and maintain the solid state.
2)Monoliths are obtained by standard preparing a Sn for analysis, so its behavior and structure depends on the previous mechanical and thermal history of Sn. Ins sample in standard quartz formsmelted and cooled under standard vacuum conditions, then Sn melt poured into a SiO2 mold to made identical samples in the form of wire or rod with a spherical surface of one edge of it, then annealed and cooled in vacuum.
3)To create the minimum of seeds by moving of H2O near of the contact Sn of spherical surface of edge with polishedor spherical surface of InSbseed.in thermostat with selected T for analysis.So, to create the minimum of seeds by moving of H2O near of contact Snwith InSb in thetermostat with ice nearly of chooses T.
4)The diagram of calibration dependence of V βSn®αSn / g4.2should be attributed to the same strictly selected T for analysis.
5)The infection V should be measured repeatedly for graphical correction of errors in a visual determination of the length of the infected area. At T, chosenfor aphase transition the impurity does not accumulate, and the concentration along the entire length is constant, which is important for analysis. For the integrity of the sample, it is possible to infect as in [10,11].You can make many measurements V βSn®αSn on length, reducing the measurement error statistically. The sections along the path of the Snwhite – dark border is measured repeatedly over time. After the end of the analyze measurement with standard remelting, the αSn is converted to βSn, especially if the analysis result must be checked by direct measurement g4,2K, which is applicable only f or metals. According to the graph for a given analysis at T V βSn ®αSn from g4,2Kfind the purity of Sn. Measures of V different samples gave 1.37 and 1.41 mm/hour, corresponded to g4,2K47 500 and 55 000. Control analyses of them give g4,2K46,800 and 55,400. Errors of 1.5% and 0.8% within the measurement accuracyof V and g4,2K. And to check the reproducibility of results in 10 standard samples, an infection V was measured on the same day in the same thermostat. The average of a value of V is 1.48 mm/ hour. A maximum deviation V valueof one sample was 1.46 mm / hour, which is 1.3%, all the others gave 1.48, 149, 1.47.
Summary
By using for the practical aims of “terrible tin plague” along with its application to obtain pure powders of a given dispersion, for further purification of high-purity tin, for growing profiled crystal of a unique material αSn even with p/n transition, simple accessible method of purity Sn analysis was created, which seemed fundamentally impossible. The accuracy and reliability of the results of the proposed method with obvious availability, accessibly and simplicity even is not complicated and complex method of residual resistance without using of liquid helium. Here is only whether the method can be considered created until it still not published and not known to researchers, for whom, and not for corrupt officials, this work was done.
Gratitude’s
The author is grateful to V. V. Ryazanov for his help in measuring g4.2K- residual resistance and for his constant interest in the work, advice and discussions and cooperation with N. G. Nikishina, R. A. Ohanyan , Efremov A.S, Boronina L.R. , Sidelnikov M.S.
For more Lupine
Publishers Open Access Journals Please visit our website:
wt u have given that
link add For more Modern Approaches
on Material Science articles Please Click Here: