Groups 4 and 15 and Organotin Condensation Polymers for The Treatment of Cancers and Viruses| Lupine Publishers
Lupine Publishers| Material Science Journal
Abstract
This short review describes the use of group 4 metallocenes, group 15
organometallics and organotin polymers in the
treatment of human cancer tumors and viruses. These metal-containing
polymers show good inhibition of all the main group solid
tumors including pancreatic, lung, brain, breast, prostate and colon
human cell lines. They also show inhibition of a variety of
viruses including zika, herpes and vaccinia viruses. Synthesis of the
polymers is rapid employing interfacial polymerization and
commercially available reactants. They offer physicians a new class of
drugs for the treatment of a variety of cancers and viruses.
Keywords: Cancer; Viruses; Interfacial polymerization; Brain cancer; Pancreatic cancer; Zika virus; Vaccinia virus; Breast cancer;
Herpes virus
Introduction
Use of metal-containing agents to treat various medical problems
is well known [1-22]. Here the focus is on activities to supply metalcontaining
polymers for the treatment of various cancers and
viruses. While we have had extensive experience with platinum and
palladium polymers for the treatment of a variety of cancers, the
current emphasis is on polymers formed by incorporation of groups
4 and 15 metals and organotin condensation polymers for the
treatment of cancers and viruses [23-41]. These two polymer types
are different with their own separate biological characterizations
[26]. For instance, the platinum and palladium polymers are
addition products and not stable for long times in solution. By
comparison, the groups 4 metallocene and organotin and group 15
polymers are condensation polymers and exhibit good stability to
over 30 weeks in solution so can be treated differently with respect
to biological and physical characterizations [26-41].
Synthesis
Synthesis occurs employing interfacial polymerization [42-
46]. It is a rapid polymerization system because high-energy
reactants are employed. These high-energy reactants are acid
halides. A typical condensation reaction has an activation energy
of about 30-40Kcal/mol whereas the activation energy for the
acid halide reactions is on the order of 20Kcal/mol. The interfacial
polymerization is employed industrially to synthesize aromatic
polyamides (nylons) and polycarbonates so industry is familiar
with the system [47,48]. These interfacial polycondensation
reactions form polymer within less than one minute in decent yield.
For the syntheses described here, commercially available reactants
are employed allowing ready reproduction and scale-up to ton
levels in a somewhat straightforward manner. Rapid stirring is
employed, generally about 18,000 rpm. This allows both the rapid
polymerizations to occur with an increase in interfacial contact area
of over ten thousand compared to non-stirred systems, and good
reproducibility. For the systems described here, the reaction vessel
is a simple glass reaction vessel, one-quart Kimax emulsifying jar,
fitted onto a Waring Blender. To illustrate the overall reactions,
products formed for the organotin polymers have a repeat unit
described as follows.
R
2SnX
2+X-R-Y-> -(-SnR
2-R-)-
where X and Y are normally Lewis bases such as alcohols,
amines, acid salts, thiols, etc. These reaction sites are often varied
for a single Lewis base such as an amino acid, shown below, that
has both acid and amine reactant sites. Examples of overall reaction
products for each of the three condensation polymer groups are
given following. Reaction between the amino acid diglycine and
dimethyltin dichloride is described (Figure 1). The polymer is
described as a poly (amine ester) with the organotin unit considered
an organic moiety such as a methylene unit in such naming. For
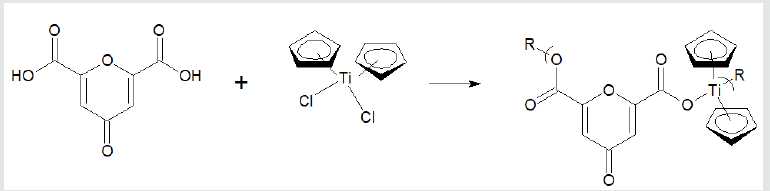
the Group 4 metallocenes, the reaction employing titanocene
dichloride as the Lewis acid, the repeat unit for a product formed
from titanocene dichloride and chelidonic acid is given (Figure 2).
Finally, for reactions involving group 15 metals, the repeat unit
formed from reaction between triphenylantimony dichloride and
3,5-pyridinedicarboxylic acid forming a polyester is given (Figure
3). The metal is generally located in the Lewis acid portion while
the non-metal reactant is the Lewis base. In certain cases, the Lewis
base portion may also contain a metal, usually iron and cobalt.
The iron is present as a ferrocene while the cobalt is present as a
cobaltocene [32].
Figure 1: Synthesis of organotin poly (amine esters) from
reaction of diglycine and dimethyltin dichloride where R represents
simple chain extension.
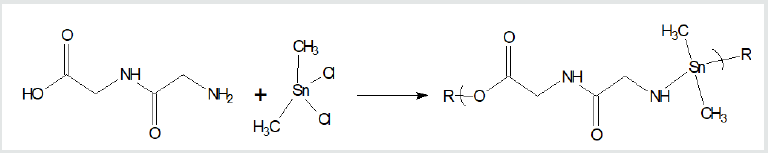
Figure 2: Synthesis of polyesters from reaction with titanocene dichloride and chelidonic acid where R represents simple chain
extension.
Cancer
It was initially mistakenly assumed that these metal-containing
compounds inhibited cancer by the same mechanism as the
platinum-containing drugs as cisplatin and other similar platinum
containing drugs [26,50]. (The platinum-containing drugs
currently are employed in over 60% of the chemo drug treatments
generally as one of the components.) It is now known that this is
not true so that they can be coupled with the drugs described here
as co-drugs that will affect inhibition of cancer through two distinct
avenues. The platinum-containing drugs are quite toxic resulting
in the presence of many negative side effects [26]. Our effort is to
create drugs that have similar or superior ability to inhibit cancer
but without the unwanted side effects. All of the metal-containing
drugs operate primarily on the DNA site for inhibition of the cancer
cell lines [26,50].
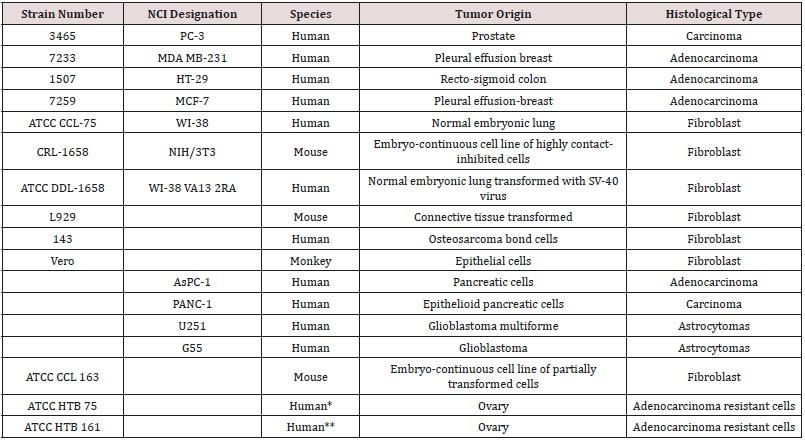
The polymers synthesized by us have shown good ability to
inhibit a variety of cancer cell lines Table 1. These cell lines
represent
all of the major human solid tumor cell lines. These cell lines include
resistant cells meaning cell lines that have shown ability to resist
treatment with the traditional anticancer drugs [39] (Table 1).
Inhibition depends on the metal atom present as well as the nature
of the Lewis base. With respect to the metal, in general, inhibition is
of the order Hf=Zr>Ti>Sn>Sb, Bi, As. Inhibition is also
dependent
on the specific Lewis base. A primary measure of the ability for a
drug to inhibit cancer growth is the effective concentration, EC. The
50% effective concentration, EC50, is the concentration of a toxicant,
drug, or antibody that induces an inhibitory response halfway
between the baseline and maximum after a specified exposure time.
The desired outcome is to have low EC50 values as this indicates that
only a small concentration of the anti-cancer agent is needed to
elicit inhibition. For the compounds described here, once inhibition
begins, the slope of the dose/concentration curve is high with
inhibition being total. Depending on the specific Lewis acid/base the
EC50 value is typically between milligrams/mL to nanograms/mL.
The metal-containing compounds are often coupled with a Lewis
base that exhibits some biological activity hoping for a syngeneic
effect. Drugs that have been employed as the Lewis bases include
ciprofloxacin, diethylstilbestrol, cephalexin, acyclovir, thiamine,
dicumarol, camphoric acid, histamine, 2-ketoglutaric acid, salicylic
acid, dipicolinic acid, isomanide, glycyrrhetinic acid, phentolamine,
thiodiglycolic acid. Lewis bases that themselves exhibit no ability to
inhibit cancer can also exhibit good inhibition when coupled with
a metal-containing moiety. These include a wide variety of diols
such as ethylene glycol, Figure 4 [29,50]. Recently, water-soluble
drugs possessing the metal-containing unit were synthesized
[29] employing as the Lewis base poly (ethylene glycol), PEG. The
resulting water-soluble polymers exhibit good inhibition of the cell
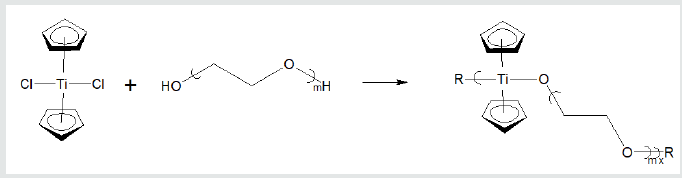
lines. Figure 5 contains the reaction between titanocene dichloride
and PEG forming water soluble polyethers (Figures 4 & 5).
Figure 3: Synthesis of triphenylantimony polyesters from reaction with 3,5-pyridinedicarboxylic acid where R is simple chain extension.
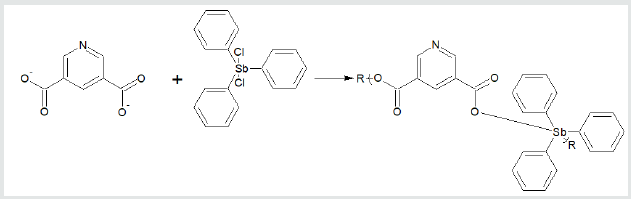
Figure 4: Reaction between ethylene glycol and dibutyltin dichloride forming polyethers.
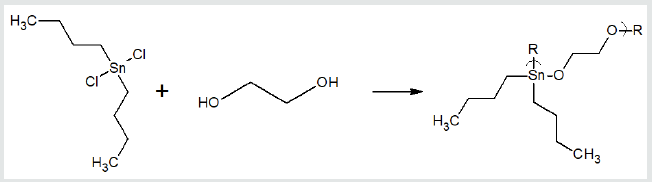
Figure 5: Formation of water-soluble polyethers from reaction of titanocene dichloride and various poly (ethylene oxides)
where R represents simple chain extension.
Viruses
These metal-containing polymers also inhibit a variety of
viruses including ones where no current drugs are available for
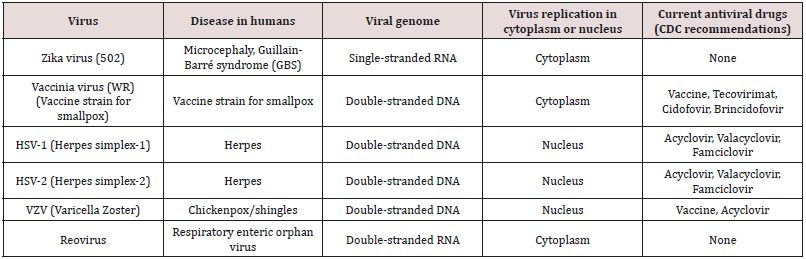
treatment [40,41,49]. Table 2 contains viruses that have been
inhibited by our metal-containing drugs including most recently the zika
virus. These viruses include both DNA and RNA viruses. They include
several that have been identified as possible weapons of mass
destruction, namely the vaccinia virus. Three DNA viruses
are effectively inhibited by the metal-containing polymers (Table
2). They are the vaccinia virus used to vaccinate humans against
smallpox; herpes simplex virus 1, the virus responsible for over
45 million infections yearly in the US, comprising one of five
adolescents and adults; and the varicella zoster virus, also a herpes
virus and responsible for chickenpox and shingles. Thus, the
metalcontaining
polymers represent a possible potent approach towards
inhibiting unwanted viruses (Table 2).
Table 1: Caner cell lines inhibited by metal-containing polymers described here.
From a cancer patient with ovarian cancer that had previously been
treated with cytoxan, adriamycin, 5-fluorouracil, and Fur IV. From
a cancer patient with ovarian cancer that had been treated with
adriamycin, cyclophosphamide, and cisplatin.
Table 2: Viruses inhibited by metal-containing polymers discussed in this report.
Why Polymeric Drugs?
A critical question is “Why Polymeric Drugs?” What
advantageousness do polymeric drugs offer [50-60]. Following
briefly describes some advantages. Each of these advantages
is related to the size of polymers and what such size offers.
First, because of their size, polymers travel through the body, in
particular the kidney and bladder, more slowly lessening organ
damage allowing the organs to limit the negative effect [50,61].
Second, cancer cells are less cohesive, offering greater porosity, and
are not as coherent as normal cells with relatively “rough” exteriors.
This allows polymers to have a greater opportunity to be “snagged”
by the cancer cells allowing them extended ability to be associated
with the cancer cells resulting in a greater ability to inhibit cell
growth. This scenario is described as the enhanced permeability
and retention effect [50,62-64]. Third, increased size allows for a
greater designing of the drug increasing its effectiveness [65-69].
This fine tuning includes attachment of “biological homing agents”.
Thus, polymeric drugs offer advantageous over small molecule
drugs that can be used to more effectively combat unwanted
diseases compared to small molecule drugs.
Summary
Metal-containing polymers show ability to inhibit all the major
solid tumor cancers as well as important viruses. They are easily
synthesized and offer physicians new drugs to attack these harmful
illnesses.














No comments:
Post a Comment